Precision medicine, or personalized medicine, is leading the way in providing a patient-focused comprehensive understanding of the science that results in effective diagnostic, prognostic, and intervention strategies.
Positively impacting human health with personalized medicine
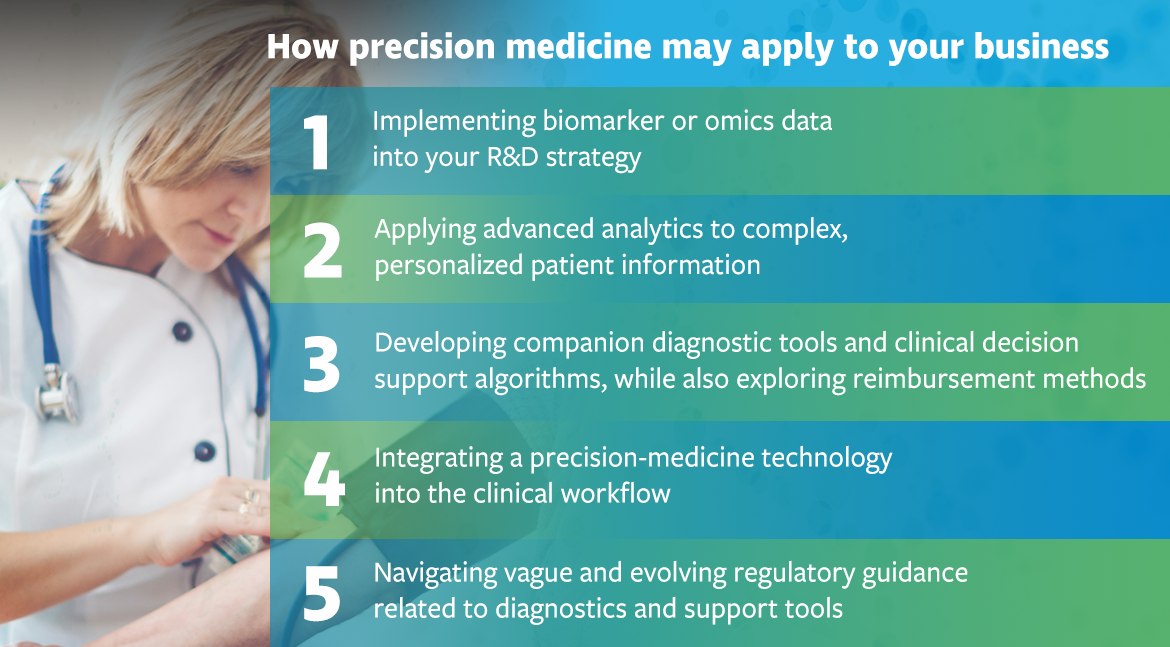
Venebio understands that precision medicine solutions must be safe and effective, while also being developed and implemented in a way that is not a burden to the clinical workflow. With a rapidly evolving area of study, our consultants guide clients through the process, eliminating the guess work in determining where to start.
Our precision medicine clients, typically pharmaceutical companies and biotech firms, work with our consultants to identify the right strategies for research surrounding drug efficacy and safety, biomarker-based companion diagnostics (CDx), immuno-oncology, medication adherence, and clinical-decision support algorithms.
Some of Venebio’s precision medicine capabilities include:
Research and development
- Market potential forecasting
- Development and review of biomarker program strategy
- Evaluation of scientific literature
- Analysis and integration of multiplatform omics data
Optimization of study design and execution
- Selection of appropriate sampling approach
- Analytical platforms, including NGS
- Biostatistical and advanced data analytic support
Clinical and regulatory strategy
- Evaluation of accuracy and robustness for clinical translation
- Development of CDx-related safety and efficacy strategy and studies
- Development of reimbursement strategies for CDx
- Safety and regulatory guidance