Venebio Opioid Advisor is built on years of research, analyzing some of the largest available databases of opioid-using patients. This work has been the focus of several published studies validating the tool’s overarching approach for stratifying at-risk patients and its efficacy at predicting a potential overdose.
Development
In response to the growing public health threat posed by the prescription opioid overdose epidemic, Venebio Group developed a predictive screening algorithm to quantitatively estimate a patient’s likelihood of experiencing life-threatening prescription opioid-induced respiratory depression (OIRD or ‘overdose’) and to characterize their associated risk factor profile. The work leading to VOA built on our previously published predictive models and scoring systems that estimate the level of risk of experiencing an adverse outcome.
Venebio Group first examined potential predictors of prescription opioid overdose in a retrospective case-control study using administrative health care claims data from almost 1.9 million U.S. Veterans Health Administration (VHA) patients who were dispensed an opioid during 2010-2012. The factors most strongly associated with experiencing an overdose included a higher total prescribed opioid dose (morphine equivalent dose (MED) exceeding 100 mg/day), diagnosis of opioid dependence, hospitalization or emergency department visit during the six months before the overdose event, liver disease, and opioids with extended-release or long-acting formulations. A risk index was then created with 15 of the variables most strongly associated with experiencing a life-threatening opioid emergency. The risk index had excellent predictive performance, with an 88% probability of correctly differentiating VHA opioid users with an overdose (N=817) and those without. There was also strong concordance between the average predicted probability of experiencing an overdose and the actual, observed occurrence of overdose by risk classes determined from the predicted probability distribution for serious overdose.
Validation
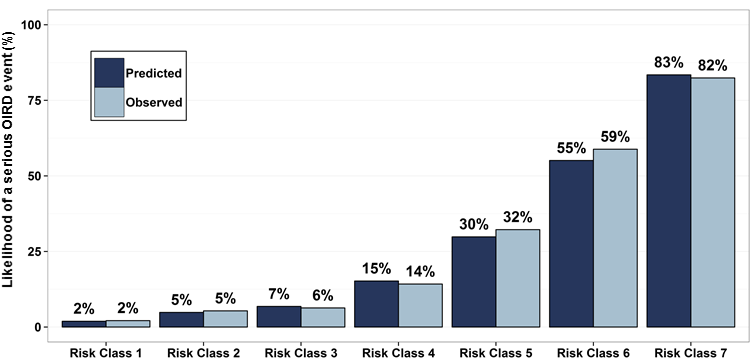
Venebio then assessed the predictive performance of the algorithm in a more representative population of U.S. medical users of prescription opioids using administrative health care claims data from 18,365,497 patients in a commercial health plan database who were treated with a prescription opioid during 2009-2013. The risk index, slightly modified based upon the variables available in the commercially insured database, showed strong performance by correctly discriminating the opioid users who experienced serious OIRD (N=7,234) and those who did not in 90% of instances. The factors most highly associated with overdose in the commercially insured population were diagnosed substance use disorder and depression, followed by other mental health disorders, impaired liver, kidney, vascular, and lung function, and non-cancer pancreatic disease. Pharmacologic factors included higher daily opioid doses, certain opioids and opioids with extended-release or long-acting formulations as well as concurrent psychoactive medications such as antidepressants and benzodiazepines. The average predicted probability of serious OIRD in the commercially insured sample ranged from 2% to 83%, when divided into risk classes by increasing predicted probability of an event, with excellent agreement between the average predicted and observed incidence across risk classes (Figure 1).
Health and Economic Outcomes
Successful implementation of VOA is intended to shift opioid-treated patients from higher risk classes to lower risk classes and decrease the incidence of opioid overdose. To evaluate health outcomes and economic impact, Venebio conducted an analysis of the aggregate effect of implementing the clinical decision support tool in a population of 100,000 patients treated with opioids. The analysis revealed that implementing VOA risk assessment and its evidence-based risk mitigation guidance can prevent, on average, more than 500 overdoses per 100,000 opioid recipients per year. This translates into preventing over 400 prescription opioid overdose-related emergency department visits and avoiding over 120 hospitalizations. The reduction in emergency department and inpatient utilization can yield more than $2 million in annual cost savings per 100,000 patients receiving opioid therapy per year.
Ongoing Research
Ongoing research is funded through a Small Business Innovation Research (SBIR) Phase 2 grant from the National Institutes of Health – National Institute on Drug Abuse (Grant ID: 1R44DA042655-01, Principal Investigator: Dr. Maciek Sasinowski). The objectives of the SBIR Phase 2 project are to: (i) refine and map the administrative claims codes for the risk factor variables and the overdose outcome from ICD-9 to ICD-10; (ii) further develop the evidence-based clinical decision support output; (iii) develop a robust and clinically useful software platform; and (iv) validate clinical utility and economic value of the refined Venebio Opioid Advisor in a series of prospective pilot studies.
VOA Publications by Venebio Group
- Nadpara P., A. Joyce, E.L. Murrelle, N.C. Carroll, N.V. Carroll, M. Barnard, B. Zedler. Risk factors for serious prescription opioid-induced respiratory depression or overdose: comparison of commercially insured and Veterans Health Affairs populations. Pain Medicine, 2017.
- Zedler B., W. Saunders, A. Joyce, C. Vick, and E.L. Murrelle. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a U.S. commercial health plan claims database. Pain Medicine, 2017.
- Zedler B., L. Xie, L. Wang, A. Joyce, C. Vick, J. Brigham, F. Kariburyo, O. Baser, and E.L. Murrelle. Development of a Risk Index for Serious Prescription Opioid-Induced Respiratory Depression or Overdose in Veterans’ Health Administration Patients. Pain Medicine, 2015.
- Zedler B., L. Xie, L. Wang, A. Joyce, C. Vick, F. Kariburyo, P. Rajan, O. Baser, and L. Murrelle. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Medicine, 2014.
Other Opioid-Related Publications by Venebio Group
- Weiner S.G., A. Joyce, H. Thomson. The prevalence of nasal obstruction as a consideration in the treatment of opioid overdose. Forthcoming in Journal of Opioid Management, 2017.
- Zedler B., A.L. Mann, M.M. Kim, H.R. Amick, E.L. Murrelle, H.E. Jones. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction, 2016.
- Wurst K.E., B.K. Zedler, A.R. Joyce, M. Sasinowski, E.L. Murrelle. A Swedish population-based study of adverse birth outcomes among pregnant women treated with buprenorphine or methadone: Preliminary findings. Substance Abuse: Research and Treatment, 2016.
- Lavonas E.J., S.G. Severtson, E.M. Martinez, B. Bucher-Bartelson, M.C. Le Lait, J.L. Green, E.L. Murrelle, T. Cicero, S.P. Kurtz, A. Rosenblum, H.L. Surratt, R.C. Dart. Abuse and diversion of buprenorphine sublingual tablets and film. Journal of Substance Abuse Treatment, 2014.
- Lavonas E.J., W. Banner, P. Bradt, B. Bucher-Bartelson, K.R. Brown, P. Rajan, E.L. Murrelle, R.C. Dart, J.L. Green. Root causes, clinical effects, and outcomes of unintentional exposures to buprenorphine by young children. Journal of Pediatrics, 2013.
- Thompson S., M. Sasinowski, B. Zedler, A. Joyce. Predictive analytics can help reduce prescription opioid overdoses and health care costs. Venebio Group Internal Report, 2017.
Full list of Venebio publications
