
All eyes are on a select number of vaccine candidates vying for Emergency Use Authorization (EUA) from the U.S. FDA. In these unprecedented times, FDA has published guidance for EUA of COVID-19 vaccines,1 and made efforts to communicate their focus on balancing safety and efficacy with urgency of approving and distributing one or more viable vaccine candidates.2
Here, we’ll review the FDA guidance for EUA of COVID-19 vaccines, and provide our favorite resources for the most current vaccine candidate updates.
Emergency Use Authorization for COVID-19 Vaccines3
As is common in all FDA guidance, the recommendations for issuance of EUA of COVID-19 vaccines are nonbinding guidelines. This allows for flexibility and discretion by both the FDA and candidate sponsors to consider and integrate the most efficient and effective means to demonstrate a vaccine’s safety and efficacy. Sponsors send study protocols and interim updates to FDA for intermediate guidance and often hold meetings together to discuss strategy and submission timelines.
One of the many unique facets of the application of a COVID-19 vaccine is its broad intended use across millions of Americans independent of age and health status. Considering this widespread and urgent application need, FDA states, “for a COVID-19 vaccine for which there is adequate manufacturing information to ensure its quality and consistency, issuance of an EUA would require a determination by FDA that the vaccine’s benefits outweigh its risks based on data from at least one well-designed Phase 3 clinical trial that demonstrates the vaccine’s safety and efficacy in a clear and compelling manner”.
A Phase 3 trial is aimed at distributing the vaccine to a large group of individuals and assessing efficacy endpoints (presumably, preliminary safety endpoints have been established during Phase 1 and Phase 2 trials). See the graphic below released from the Centers for Disease Control and Prevention for a refresher on the typical clinical trial development pathway.4 Note that a typical vaccine takes many millions of dollars to develop and distribute across ten to fifteen years,5 making these expedited COVID-19 vaccine development timelines quite extraordinary.
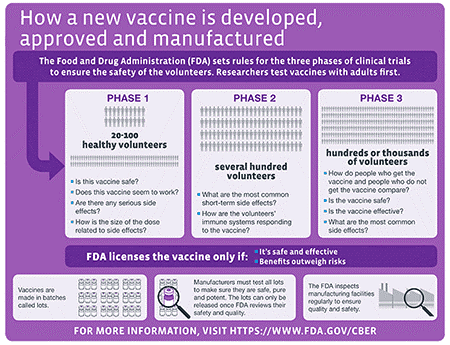
In order for a vaccine to be considered for EUA for COVID-19, the FDA has stated that the sponsor should provide the following in their submissions:
- Chemistry, manufacturing and controls involved in vaccine development
- Investigational new drug (IND) package reviewing pre-clinical testing of the vaccine
- Proposed use, dosing regimen, and methods of administration
- Safety and efficacy information
- Specific information related to use in adult, pediatric, geriatric, pregnant/lactating, and immunodeficient individuals
- Risk benefit assessment including steps taken to mitigate risk and optimize benefit, restrictions to ensure safe use, and considerations prior to use or situations in which the product should not be used
- Draft package inserts and fact sheets
- Supply chain information reviewing manufacturing and availability
As is always the case, applicants will need to provide manufacturing facility information, generate a quality control system to ensure consistent and sterile manufacturing and provide a stability and storage plan to ensure the vaccine remains chemically stable over time. An adverse event detection and mitigation plan must also be provided.
Often overlooked is the way in which the applicant will demonstrate that the vaccine is effective through their clinical endpoints and any diagnostic assays. This is especially critical during quick-turnaround applications where long-term endpoints may not be gathered and proxy endpoints may be used instead (e.g., assessing antibody production rather than observing the vaccine patient over time to determine whether they avoided contracting the virus or experienced a reduced severity disease course). The applicant will need to provide information justifying their selected outcomes.
How long will the process take?
In order to remain sensitive to the urgency of the development and deployment of a COVID-19 vaccine, FDA suggests that Phase 3 trials extend at least two months. Phase 1 and Phase 2 trial data can be applied to observe outcomes and adverse events as they will have begun earlier than Phase 3 trials. Still, the Phase 3 trials must demonstrate any local and systemic adverse reactions across all age cohorts, adverse events for at least one month after completion of the vaccine regimen in at least 3,000 patients, and at least five cases of severe COVID-19 in the placebo group. Attainment of these milestones will impact the amount of time required until Phase 3 trial activity is sufficient enough for an EUA.
Once an EUA is submitted, the FDA has 30 days to respond to the application. They may approve the EUA, reject it, or respond with questions, which initiates a sometimes lengthy communication process between the FDA and the sponsor.
After a candidate is approved for EUA, a massive manufacturing, storage and distribution effort must be undertaken in order to deploy the vaccine across the U.S. Federal and regulatory bodies have acknowledged this burden, and are attempting to mitigate some of these costs with federal funding initiatives including Operation Warp Speed (OWS).6 The Biden administration has also announced intentions to invest approximately $25 billion in vaccine manufacturing and distribution.7
Where do we stand now?
Five vaccine candidates are currently in Phase 3 clinical trials in the U.S. with intentions to submit to FDA for EUA.
- Moderna in partnership with National Institutes of Health: A two-dose regimen which requires storage in a deep freeze until injection. Phase 3 trials began July 27, and finished recruitment of 30,000 participants in October. The U.S. government will award $1.5B for 100M doses if the FDA grants EUA.
- Pfizer in partnership with BioNTech and Fosun Pharma: A two-dose regimen which requires storage in a deep freeze until injection. Phase 2/3 combined trials began July 27, and expanded their participant recruitment goals to 43,000 in September. In October, they received permission to test the vaccine in participants 12 and older. While waiting to observe enough cases of COVID-19 to determine if the vaccines are efficacious, a November 8 analysis showed promising results of the first 94 cases indicating 90% efficacy in participants who received the vaccine.8,9 The U.S. government will award $1.9B for 100M doses if the FDA grants EUA.
- Johnson and Johnson in partnership with Beth Israel Deaconess Medical Center: A one-dose regimen. Phase 3 trials resumed late October after a brief pause to investigate an adverse reaction. The U.S. government will award $1B for 100M doses if the FDA grants EUA.
- AstraZeneca in partnership with the University of Oxford: Phase 3 trials resumed in the U.S. on October 23 after a brief pause when a participant experienced adverse reactions. The U.S. trial funded by BARDA covers 30,000 participants. The U.S. government will award $1.2B for 300M doses if the FDA grants EUA.
- Novavax in partnership with the Coalition for Epidemic Preparedness Innovations: Expecting to launch a Phase 3 trial in the U.S. by end of November. The U.S. government will award $1.6B for if the FDA grants EUA.
Our scientists follow these publicly available resources for COVID-19 vaccine updates:
1. FDA
2. FDA
3. FDA
4. CDC
5. History of Vaccines
6. HHS
7. Build Back Better
8. New York Times
9. Pfizer
